- info@clinicalresearchofontario.com
- +1 (416)-648-6167
- Fax: (416) 332-8081
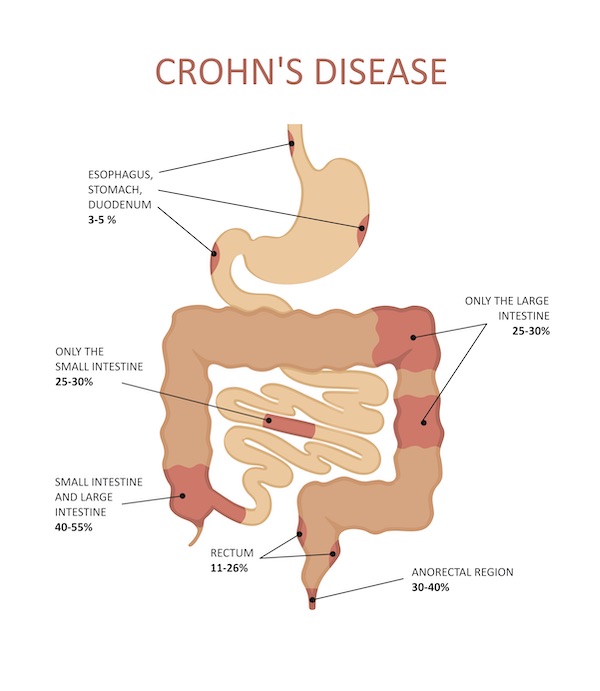
Crohn Disease (CD) can be broadly defined as an inflammatory disease affecting any part of the GI tract from mouth to anus, though it commonly affects the ileum and colon. Importantly, CD is characterized by the involvement of the entire gut wall, with an anatomical location in any part of the GI tract and a tendency to present complications such as fistulae and strictures. There are several theories as to contributing factors for CD pathogenesis. The complex etiology includes an interplay between genetics, bacteria, and environmental factors.
Patient compensation is available for time.
Clinical research are research studies that are performed in patients to evaluate medical treatment and procedures. The goal of clinical research is to improve the diagnosis and treatments of patients and to improve the health of individuals who participate in the trials. Clinical trials help determine the safety and efficacy of medications, vaccines, diagnostic products and medical devices to all types of disease