- info@clinicalresearchofontario.com
- +1 (416)-648-6167
- Fax: (416) 332-8081
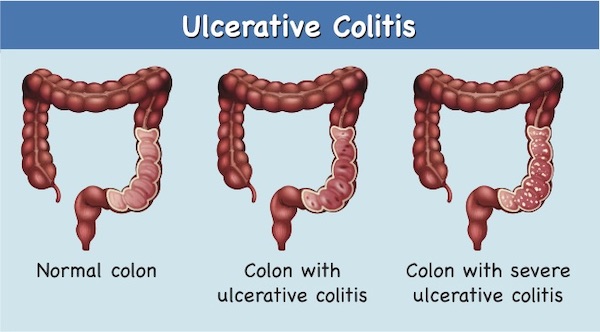
Ulcerative Colitis (UC) manifests through complex interactions between the gut microbiome, dysregulated immune responses, genetic mutations, diet, and other environmental factors. As a result, the precise stimulus for the initiation of disease differs widely among patients with UC. Current therapies have limited efficacy and significant side effects. An unmet clinical need remains because CD and UC are not well managed pharmacologically with current drugs.
Patient compensation is available for time.
Clinical research are research studies that are performed in patients to evaluate medical treatment and procedures. The goal of clinical research is to improve the diagnosis and treatments of patients and to improve the health of individuals who participate in the trials. Clinical trials help determine the safety and efficacy of medications, vaccines, diagnostic products and medical devices to all types of disease